|
Correlation between the cardiac action potential in the surface electrocardiogram Cardiac myocytes are excitable cells and in response to a stimulus they can generate an action potential (AP) associated with a contractile response. An AP is a reversible change of the membrane potential caused by a sequential activation of several ion currents generated by the diffusion of ions through the membrane downs their electrochemical gradient. Thus, during the cellular depolarization the resting membrane potential moves from negative values (-85 mV) to positive values (up to +20 or +30 mV) and then recover back to the basal resting membrane potencial during the repolarizing process.
During phase 3 the repolarization is accelerated due to the inactivation of INa and ICa and the subsequent dominance of K+ currents activated repolarizantes during phase 2. At the end of phase 3 three inward-rectifier K+ currents are activated. They conduct K+ currents more in the inward direction than the outward direction and play an important role in setting the resting potential close to the equilibrium potential for K+ (EK, approximately - 90 mV) and in the repolarization of the AP. These currents are:
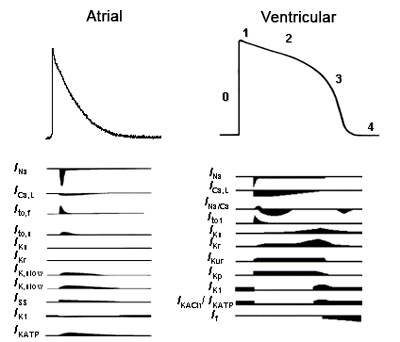
Figure. Ionic currents involved in the genesis of the atrial/ventricular AP. Tamargo y cols., 2004.
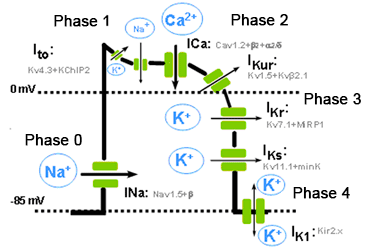
Figure. Schematic representation of the different phases of a ventricular AP, the different inward and outward ionic currents and the a subunits and the auxiliary subunits that form the various channels. The resting membrane potential during the diastole (phase 4) in non-automatic cells is isoelectric. The concentration gradients for Na+ and K+ across the plasmalemma are restored by the Na+ pump, the (Na+ -K+-ATPase), which extrudes 3 Na+ in exchange for 2 K+. Because of its electrogenic nature, the (Na+ -K+) -ATPase generates a hyperpolarising outward current that contributes to the resting membrane potential. Calcium entering the cell during the plateau phase is removed by the Na+ -Ca2+ exchanger (NCX1), which exchanges 3 Na+ for 1 Ca2+. The direction of movement of these ions (inward or outward) depends upon the membrane potential and the chemical gradient for the ions. When the membrane potential is negative (i.e., during phases 3 and 4 of the AP), the NCX1 transports Ca2+ out as Na+ enters the cell, while when the cell is depolarized (phases 0, 1 and 2 of the AP), the exchanger works in the opposite direction (i.e., Na+ leaves and Ca2+ enters the cell). Thus, NCX1 also contributes to Ca2+ entry during the plateau phase of the AP. Correlation between the cardiac action potential and the surface electrocardiogram The phases of the cardiac action potential correspond to the surface ECG (ECG) (Figure). The P wave reflects atrial depolarization (phase 0), the PR interval reflects the conduction velocity through the AV node, the QRS complex the ventricular depolarization and QT interval the duration potential ventricular action. Gradients in ventricular repolarization are reflected in the T wave. Widening of the QRS complex reflects reduced intraventricular conduction velocity, which typically results from altered Na+ channel function. ST segment elevation reflects transmural voltage gradients during the AP plateau, a hallmark of the Brugada syndrome.
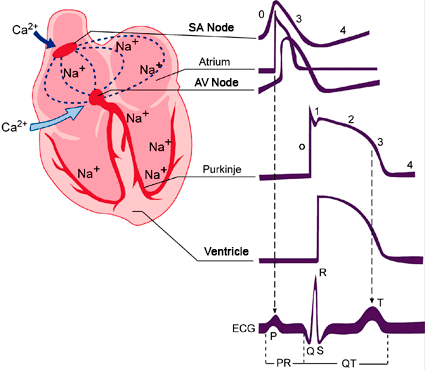
Figure. Schematic representation of the action potentials recorded in various cardiac tissues and its correlation with the surface electrocardiogram (ECG). T issues that generate Ca2+ - dependent (SA and AV nodes) and Na+ - dependent action potentials (atria, ventricles and His- Purkinje system. SA: sinoatrial node. AV: atrioventricular node.
Electrical excitation in the human heart originates in specialized pacemaker cells located in the sino-atrial (SA) node. The AP of these cells do not present an isoelectric diastolic potential but a spontaneous slow diastolic depolarization, which depolarizes the membrane potential back towards the excitation threshold generating rhythmic AP. The rate of this diastolic depolarization determines the rate and rhythm of the normal heartbeat.
When the membrane potantial of the SAN cells reach the maximum diastolic potential, the K+ conductance decreases and inward depolarizing currents (such as If and ICaL) increase, channels long believed to be the single major pacemaker inducing an spontaneous diastolic depolarization. The depolarization-induced Ca2+ entry facilitates the release of Ca2+ from the SR generatin a Ca2+ signal that activates the INCX. This results in NCX-mediated Na+ influx leads to a net gain of positive charges and membrane depolarization during late diastolic interval and accelerates the time for reaching the threshold potential.
|
| Aviso legal Esta obra está bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional |