|
Ion Channels The cell membrane barrier separates two aqueous media of different composition, the extracellular and intracellular spaces, regulating their composition. The most lipophilic drugs and solutes when not ionized travel directly through the cellular membrane via a passive diffusion process, which facilitates the passage of a substance from an area of high concentration to an area with lower concentration. The difference of concentration between the two areas is often termed as the concentration gradient, and diffusion will continue until this gradient has been eliminated. The speed of this process will be more rapid the higher the concentration gradient and the lipophilicity of the molecule and smaller the size of it (Fick's law).
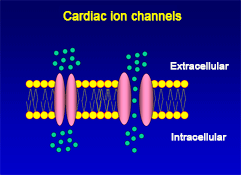
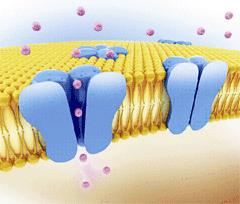
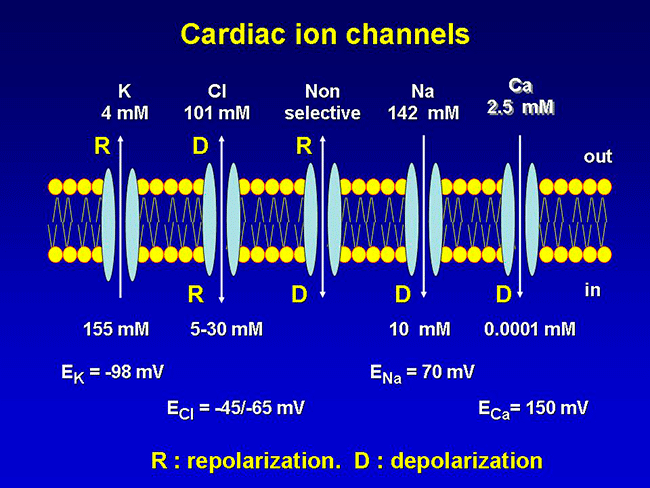
The cellular action potential which the beating heart to contract, is the result of a series of sequential reversible changes in the conductance of the membrane to different ions produced in response to changes in the electrical potential between cell and the surrounding environment. Ions are hydrophilic molecules that cross the hydrophobic lipid bilayer through specialized structures called ion channels.
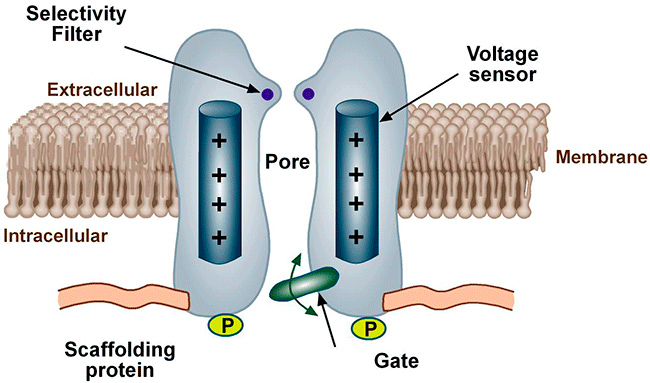
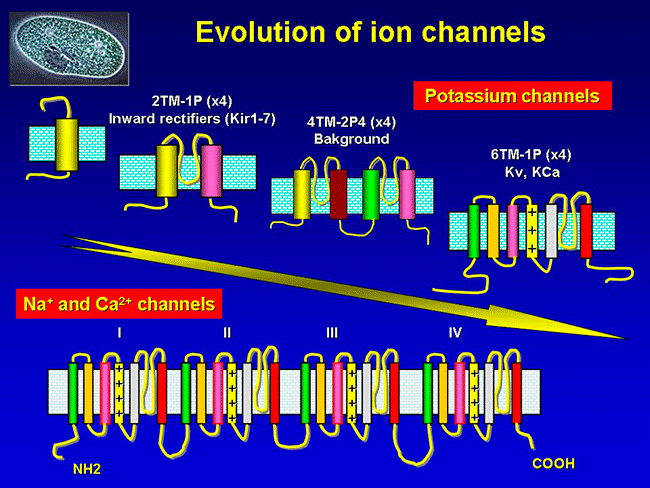
Figure. Ion channels are pore-forming proteins that provide pathways for the controlled transmembrane movement of ions However, the ion channel expression is not restricted to the sarcolemma of excitable cells, but they can also be expressed in the membranes of some intracytosolic organelles (i.e., the sarcoplasmic reticulum, mitochondria). Channels located in the sarcoplasmic reticulum play a key role in cellular Ca2+ handling. Gap junctions are aggregates of intercellular channels that allow direct communication between adjacent cells through the diffusion of ions, metabolites, and small cell signaling molecules. The intercellular channels are formed by head-to-head docking of hexameric assemblies (connexons) of tetraspan integral membrane proteins, the connexins (Cx) Cell-cell communication mediated by connexins is crucial in the propagation of cardiac impulses. Cardiac Na+, Ca2+ and K+ result from the coassembly of a pore-forming a subunit with other"accesory or auxiliary" subunits. However, the ion channels are not simple aqueous pores conductors, but, present (Figure):
According to the stimulus that induces the opening of cardiac ion channels are classified as:
However, quite often this division of the ion channels is artificial, because the depolarization of the membrane can also induce the release of endogenous ligands and open channels activated by receptors or intracellular mediators, whereas many endogenous ligands can also modify the membrane potential and activate voltage-gated channels.
Conformational states of the ion channels The voltage-gated channels present, at least, a conductive state (open-O or active state) and two non-conductors (inactive-I and resting-R states). The R state does not allow passage of ions, but channels can open from the R state in response to specific stimuli. The O state allows passage of ions across the cell membrane which generates an ionic current. When we apply a depolarizing pulse voltage-gated chanels move from the R to the O state, i.e. channel activation. However, if the depolarization is maintained, the open channel probability decreases as a result of the inactivation process initiated simultaneously by the activation process. The channel then goes into an I-closed state from which the channel can not be reopened until it returns to the R state. For the opening takes place the channel should return to idle. This step in the inactive state of rest, is called channel reactivation and occurs during cell repolarization. Therefore, the magnitude of the current which crosses the membrane depends on the density of channels, open channel conductance and how long the channel remains in the open state.
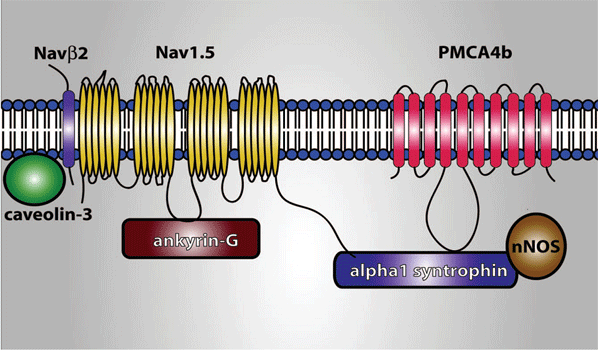
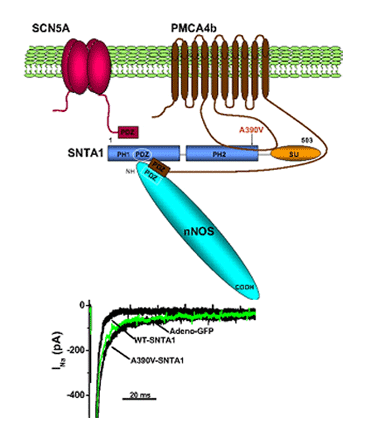
Until recently, it was considered that the cardiac ion channels were heteromultimeric complexes were formed by the assembly of the a subunit with one or more auxiliary subunits. However, in recent years it has become evident that even when the coassembly of these subunits can form a functional channel, in most cases the correct operation of the channel requires its specific location in a given area of the sarcolemma, its anchoring to the cytoskeleton and/or its binding to scaffold and signalling proteins that associate the channel with other channels, receptors or enzymes. This set of proteins constitute the channelosome and represent the structural and functional unit of the ionic channel. The sodium channelosome is shown in Figure.
Hille B. Ion channels of excitable membranes. Hille B (Ed.). 2001. Payandeh J, Scheuer T, Zheng N, et al. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353-358. Cardiac Electrophysiology. From cell to beside. Eds. Zipes DP, Jalife J. Elsevier. Estados Unidos. Sixth Edition. 2013. |
| Aviso legal Esta obra está bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional |